Research in Hypervalent Iodine Chemistry
Most people are familiar with the element iodine due to its popularity as an antiseptic sold as iodine tinctures. These alcohol containing solutions cause iodine to have a brown color through the generation of something called a charge transfer complex. If iodine is not in an alcohol solution, if forms a different charge transfer complex with air that makes it take on a purple color. However, when in a vacuum, iodine looks like a silvery metal (see pictures on the right). Iodine is an immensely diverse element and exploring the metal-like properties of compounds containing iodine in its hypervalent state is the main focus of the Hyatt Research Group.
When chemists call something "hypervalent" it means that the normal rules of bonding do not apply. Typically, nonmetals follow the octet rule; they prefer to form structures with eight shared electrons around each element. Hypervalency is when compounds form with over eight electrons. In our group, we use iodine(III) which has 10 electrons in most structures. In this higher oxidation state, the hypervalency can be used to exploit unusual and often unexplored metal-like properties of this commonly encountered nonmetal.

Publications with Adelphi University Undergraduates
Vanie Seecharan, Lyse Armand, Jennifer N. Noorollah, Nirvanie Singh, Andrew Zhang, Kevin P. Freddo, Nicholas Spatola, Sailesh Prasad, Azka Chaudhry, Su Wint War, I. F. Dempsey Hyatt, and Daniel L. Silverio, ARKIVOC, 2021, 79-85. Ligand exchange of aryl iodine dicarboxylates to form reagents with differing solubilities
Taro Jones, Jennifer Noorollah, Nirvanie Singh, Nicholas R. Spatola, Andrew Zhang, Azka Chaudhry, and I. F. Dempsey Hyatt, ARKIVOC, 2020, 59-66. Computational study of phenyliodine diacetate intermediates during Lewis acid activation with TMSOTf
Jennifer Noorollah, Haram Im, Fatima Siddiqi, Nirvanie Singh, Nicholas R. Spatola, Azka Chaudhry, Taro J. Jones, and I. F. Dempsey Hyatt, European Journal of Organic Chemistry 2020, 2302–2305. para-Selective benzylation of aryl iodides via the in situ preparation of ArIF2: a hypervalent iodine-guided electrophilic substitution
I. F. Dempsey Hyatt, Loma Dave, Navindra David, Kirandeep Kaur, Marly Medard, Cyrus Mowdawalla, Organic and Biomolecular Chemistry 2019, 17, 7822-7848. Hypervalent iodine reactions utilized in carbon-carbon bond formations
Cyrus Mowdawalla, Faiz Ahmed, Tian Li, Kiet Pham, Loma
Dave, Grace Kim, I. F. Dempsey Hyatt, Beilstein
Journal of Organic Chemistry 2018,
14, 1039-1045. Hypervalent iodine-guided
electrophilic substitution: para-selective
substitution across aryl iodonium compounds with
benzyl groups
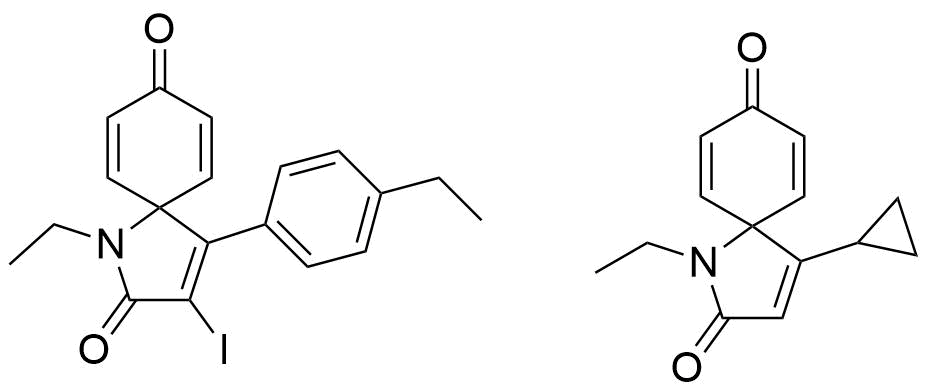
Formation of
Spirolactams for Trichomonas Vaginalis
Hypervalent iodine undergo dearomatization to
obtain core structure of spirolactams which is
evaluated for testing of inhibition of enzymes
for Trichomonas vaginalis.
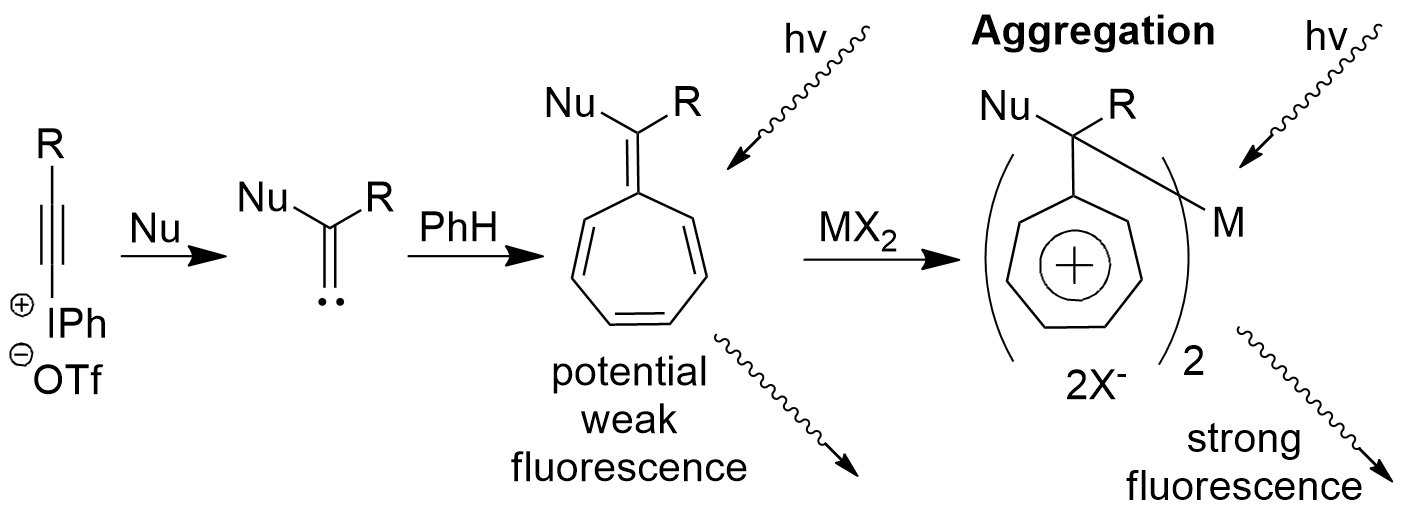
Aggregation Induced Emission of Cycloheptatrienylidenes
Hypervalent Iodonium Alkynyl Triflates (HIAT) undergo a ring-expansion reaction with a benzene ring to synthesize cycloheptatrienylidene (CHT). The molecule is then treated with a metal ion to initiate a aggregation that induces fluorescence.
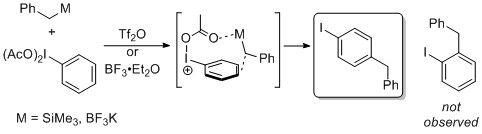
Learn moreHypervalent
Iodine-Guided Electrophilic Substitution
(HIGES)
The metal-like properties of hypervalent
iodine was used in a transmetallation
mechanism with metalloid groups containing
silicon and boron and triflate-based
activators to form para-selective
products with benzyl hypervalent iodine
intermediates.