Chakraborty's
Laboratory
Neuroendocrinology
Research Projects
Teachings
Current students/ Alumni
Publications
Meetings and Presentation
Animal Facility
Project
1: ‘Hypoglycemia And Estrogen’
Model: Hypothalamic cell line
Hypoglycemia
is a condition when the blood glucose level falls below normal levels.
Glucose is the main metabolic fuel of the brain. Recurrent episodes of
hypoglycemia can lead to seizures, coma, and even death. There is
evidence that estrogen, a steroid hormone, mainly produced by ovaries,
is required for the maintenance of glucose homeostasis.It
has been suggested that
estrogen affects cell viability, survival and proliferation and
regulates genes positively and negatively through intracellular
signaling of membrane receptors (e.g. G- protein, Ca++, cAMP,
PKC,
etc) where the receptors are outside the nucleus in the cytoplasm or
the plasma membrane; the nongenomic pathway or membrane initiated
steroid signaling.. However, the molecular mechanism involving the
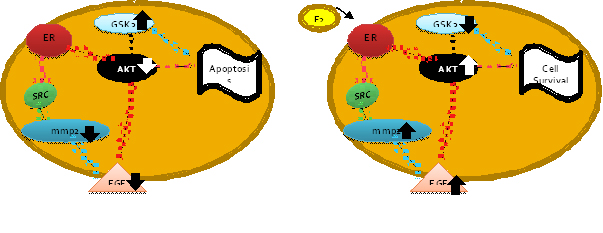
event is a subject of debate. We propose that estrogen exerts its
neuroprotective effect through the AKT-GSK3b signaling pathway since
our DNA microarray data suggest that AKT is downregulated in the
absence of glucose and in the presence of estrogen thre is an
upregulation promoting cell survival.
Experimental
Questions1. Does hypoglycemic injury trigger apoptosis in subpopulations of hypothalamic neurons through specific death pathways? Is estrogen able to provide protection against injury?
2. Is the neuroprotection of estrogen mediated through ERalpha or plasma membrane receptor?
3. Are there caspase induced neuronal cell death?
Project 2: ‘Atrazine
as an Endocrine Disrupting Chemical’
Project 3: ‘Effect of estrogen and leptin in the estrous cycle of ob/ob and ovariectomized mice’
Model: ob/ob and Ovariectomized Mice
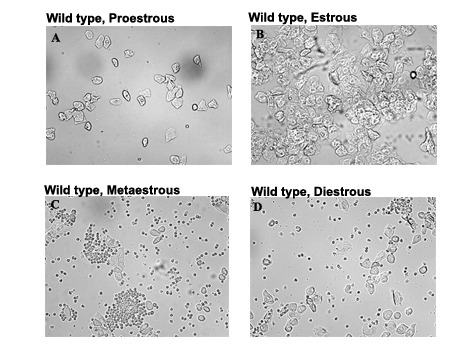
Estrous cycle is the recurring physiological changes occurring in the endometrium of the uterus during the reproductive cycle. There is a known impact of hormones like estrogen and leptin on the regulation of the estrous cycle. Estrous cycle in mice is a 4-5 day cycle characterized by proestros, estrous, metaestrous and diestrous, which can be determined by the cell types obtained from the vaginal smear. Analyzes of estrous cycle is an important tool as female mice are considered to be an excellent model for the study of neuroendocrine mechanism underlying a wide variety of normal functions and pathophysiological conditions in human. Estrogen, a hormone secreted mainly by the ovaries not only regulates the reproductive cycle but also has a negative effect on food intake. Leptin, a hormone secreted by the fat cells has an inhibitory effect on food intake. In this study we tried to analyze the estrous cycle in relation with leptin and estrogen levels in two mouse lines ob/ob and ovariectomized (OVX) female mice, both of which are infertile, increased body weight, with low or no estrogen but with contrasting leptin levels.
Experimental Questions
1. How does that estrous cycle of ob/ob and OVX mouse vary among these animals?
2. Whether there is any effect of estrogen and leptin on the estrous cycle?
Project 4: ‘Regulation of Feeding Circuits by Hormones and Neuropeptides and Menopause’.
Model: Ovariectomized mice and Wild-type mice
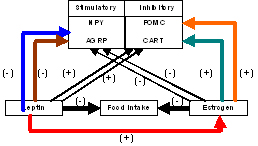
Reproduction and feeding are closely tied and are under the tight control of the hypothalamic-pituitary axis. Menopause, a process that usually occurs with age, there is a precipitous decline in circulating estrogen levels, tends to be associated with an increased risk of obesity and health risks that leads to increased insulin resistance, dyslipidemia, high blood pressure, diabetes, heart disease and hypertension. Estrogen, a hormone secreted by the ovaries and leptin an hormone secreted by the fat cells is the main focus of this study. Our hypothesis is that estrogen and estrogen receptor (ER) signaling pathways play an important role in the regulation of feeding and energy expenditure by interaction with hypothalamic neural pathways. Our preliminary data indicate that ovariectomy in mice is associated with an increase in the number of ER alpha (ER)-expressing cells in the hypothalamic arcuate nucleus (ARH) compared to wild type females, leading to changes in leptin receptor (LR) levels several months following surgery. Moreover, leptin inhibits food intake by inhibiting other proteins involved in the feeding circuit like neuropeptide Y (NPY)/ agouti-related protein (AGRP) and proopiomelanocortin (POMC)(see figure). It is thus possible that a decrease in circulating sex steroids leads to an alteration in leptin responsiveness and there lies a common signaling pathway for both leptin and estrogen. However, the mechanisms of this interaction are largely unknown, yet a better understanding of these circuits offers the potential for direct therapeutic intervention to reduce obesity and the significant morbidity that is associated with it in postmenopausal women.
 The objective of our proposal
is to define the role that estrogen, ER and neuropeptide signaling play
in the hypothalamus to influence energy homeostasis during menopause
using follicle-depleted and ovariectomized mice (OVX) as model systems.
This will be accomplished as follows:
The objective of our proposal
is to define the role that estrogen, ER and neuropeptide signaling play
in the hypothalamus to influence energy homeostasis during menopause
using follicle-depleted and ovariectomized mice (OVX) as model systems.
This will be accomplished as follows:Experimental Questions:
1. How are the neuropeptide phenotypes (e.g. NPY/AGRP, POMC) of neurons in specific regions of the hypothalamus that are ER positive, both in normal and estrogen-deficient female mice regulated?
2. Determine whether the neuropeptides (e.g. NPY/AGRP, POMC) colocalize with ER and the effect of estrogen-deficiency on their gene expression in the ARH and whether estrogen treatment alters the expression and circuits in any way?